Anode for Na-ion batteries
An extremely cheap anode made from red phosphorus and carbon xerogel.
This project aims at anode material for sodium-ion batteries with extremely low cost. We report a method to condense red P on carbon xerogel to synthesize anode with low cost, high capacity, and good capacity retention. Even with large particle size (~ 50 μm) and high mass loading (2 mg cm-2), the composite cycled at 100 mA g-1 yielded a capacity of 357 mA g-1 (mass calculated from composite), or 2498 mAh gP-1 based on phosphorus mass after subtracting the contribution of carbon. The average coulombic efficiency is as high as 99.4%. When cycled at 200 mA g-1, it yielded a capacity of 242 mA g-1 or 1723 mAh gP-1, with average degradation rate only 0.06% in 80 cycles.
Background
Sodium ion batteries (SIBs) have been attracting much attention due to abundant resources of precursors and the potential low cost. Phosphorus has a great potential to serve as the anode of sodium ion batteries owing to its high theoretical capacity (2596 mAh g-1). However, the large radius of sodium ion makes it difficult to achieve and maintain high capacity after cycling. Various methods have been attempted to increase the capacity of phosphorous anode. Almost all of succesfully ones are composed of very expensive materials such as black phosphorus or graphene, losing the low-cost advantage of sodium-ion batteries. We want to come up with a synthesis procedure with extremely low cost and comparable performance with state-of-the-art methods.
Methods
We mixed resorcinol with formaldehyde to form resorcinol-formaldehyde gel. After carbonizing the polymer at 1000°C, we obtained carbon xerogel (CX). Carbon xerogel was sealed with red P in vacuum and heated up to 900°C to condense phosphorus vapor on the carbon skeleton to produce P/carbon xerogel composite (P@CX).

Results
TGA. We use thermogravimetric analysis (TGA) to measure the mass ratio of phosphorus in the P@CX composite. Assuming that all phosphorus would evaporate when the temperature reached around 400 °C, we heated the samples till 500 °C which was sufficient to cover the range of interest. From a below, we determined mass ratio of P to be 1-87.96%/(1-1.34%)=10.85%.
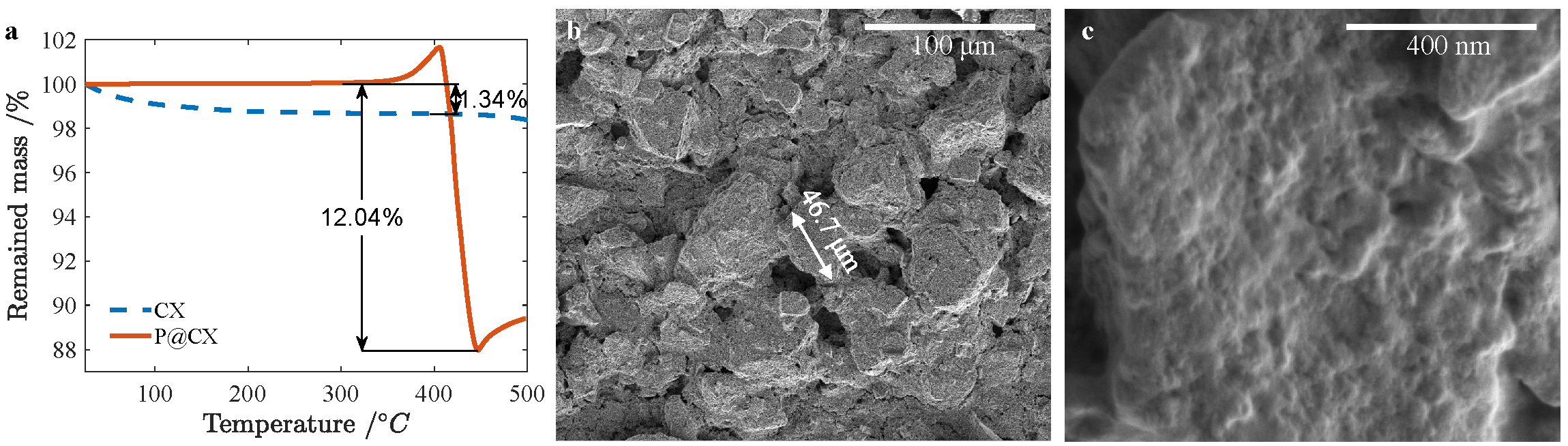
SEM. SEM images in b and c above show a porous microstructure. We can observe that the particle sizes are fairly large: some particles are about 50 μm in diameter.
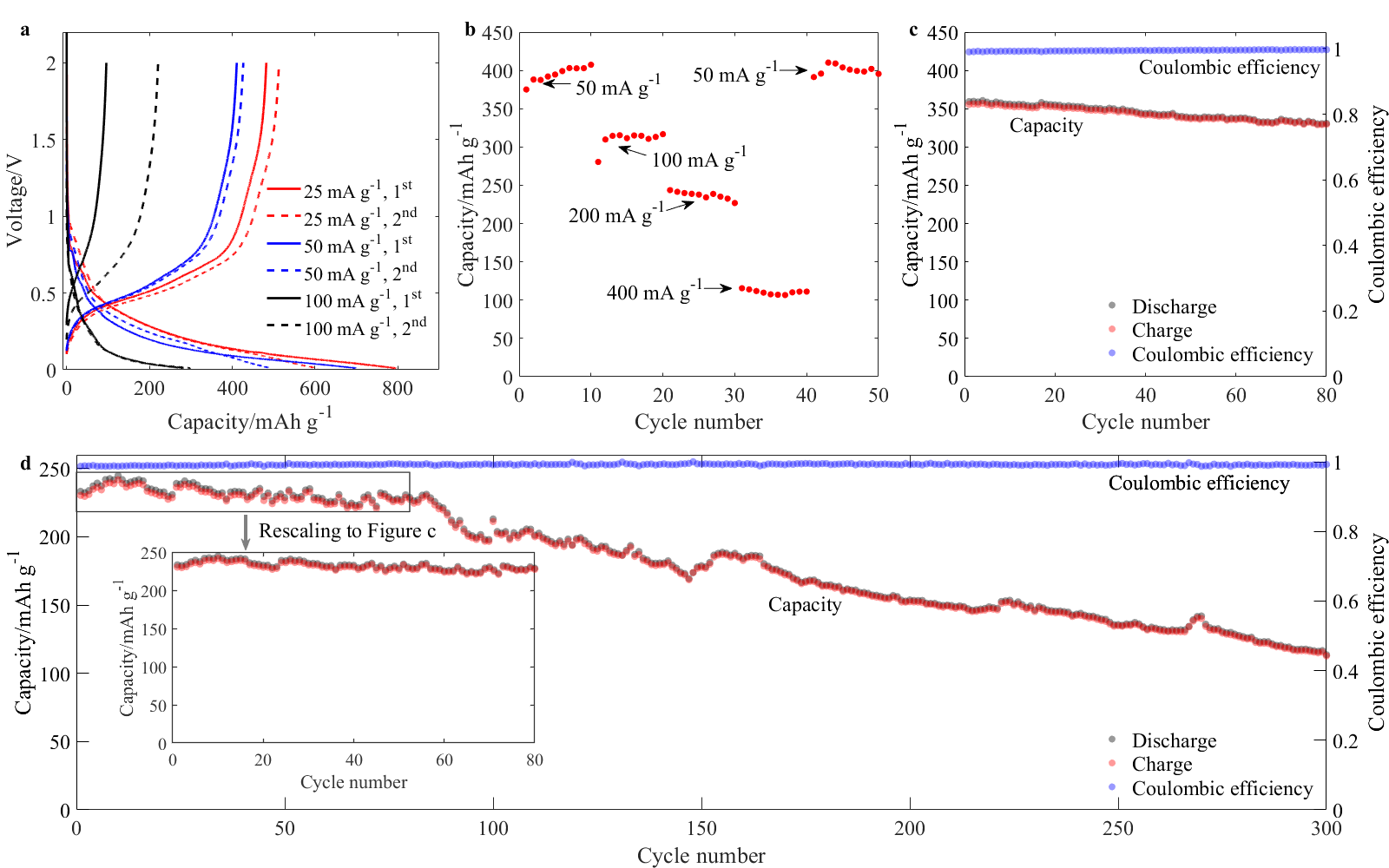
Capacity. As shown in the figure below, the reversible capacity is around 357 mAh g-1 at 100 mA g-1 and 242 mAh g-1 at 200 mA g-1, with coulombic efficiency as high as 99.4% and 99.2% respectively. Subtracting the contribution of carbon, the capacity of P was estimated to be 2498 mAh gP-1 and 1723 mAh gP-1 at current densities of 0.92 A gP-1 and 1.84 A gP-1, respectively. From the results we can conclude that carbon xerogel could help phosphorus to achieve high capacity.
Video
Presentation recording in 239th ECS meeting. It is recorded before getting all the results; some figures in this page are not in the video.